Results for 'All Articles'





Evaluation of Trials Comparing Single-Enantiomer Drugs to Their Racemic Precursors: A Systematic Review
May 6th • 18 mins read

Revamping the ever-changing landscape of drug development processes in the midst of COVID-19 pandemic
Apr 29th • 2 mins read

Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials
Apr 13th • 10 mins read

Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring
Apr 20th • 8 mins read

Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006-2020
Apr 20th • 7 mins read
Comparative study on anticancer drug access times between FDA, EMA and the French temporary authorisation for use program over 13 years
Apr 7th • 12 mins read

Virtual Clinical Trials in Oncology-Overview, Challenges, Policy Considerations, and Future Directions
Apr 8th • 4 mins read
Advances in basic research in oncology in 2020: Bridging basic science and clinical care
Mar 24th • 1 min read

Are Quality of Randomized Clinical Trials and ESMO-Magnitude of Clinical Benefit Scale Two Sides of the Same Coin, to Grade Recommendations for Drug Approval?
Feb 11th • 3 mins read

Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
Feb 23rd • 8 mins read

Assessment of Coverage in England of Cancer Drugs Qualifying for US Food and Drug Administration Accelerated Approval
Feb 22nd • 10 mins read

The First 2 Years of Biosimilar Epoetin for Cancer and Chemotherapy-Induced Anemia in the U.S.: A Review from the Southern Network on Adverse Reactions
Mar 12th • 7 mins read

Assessment of Food and Drug Administration- and European Medicines Agency-Approved Systemic Oncology Therapies and Clinically Meaningful Improvements in Quality of Life: A Systematic Review
Feb 11th • 4 mins read
Accelerated drug approvals in oncology: Pros and cons
Sep 14th • 4 mins read
Finding the Right Drug at the Right Dose the First Time: Has the Era of Personalized Formularies Finally Arrived?
Sep 27th • 1 min read
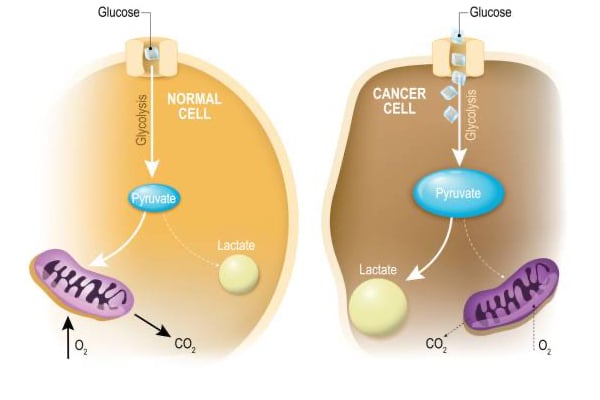
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read
Loose Regulatory Standards Portend a New Era of Imprecision Oncology
Dec 1st • 4 mins read
Confounding factors in exposure–response analyses and mitigation strategies for monoclonal antibodies in oncology
Nov 20th • 12 mins read
A narrative review of biosimilars: a continued journey from the scientific evidence to practice implementation
Aug 3rd • 10 mins read


Safeguarding cancer research funding by European charities amidst the COVID-19 pandemic
Nov 22nd • 3 mins read

Seven decades of chemotherapy clinical trials: a pan-cancer social network analysis
Oct 16th • 12 mins read

Advances and Challenges in Pediatric and Childhood Cancers
Jun 27th • 2 mins read
Liquid biopsy in oncology: a consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology
Sep 26th • 17 mins read

Leveraging existing data to contextualize phase II clinical trial findings in oncology
Sep 21st • 3 mins read
A Field Test of Major Value Frameworks in Chemotherapy of Nasopharyngeal Carcinoma-To Know, Then to Measure
Aug 12th • 10 mins read

Application of the ESMO-Magnitude of Clinical Benefit Scale (V.1.1) to the field of early breast cancer therapies
Sep 6th • 20 mins read
Lessons learnt from scoring adjuvant colon cancer trials and meta-analyses using the ESMO-Magnitude of Clinical Benefit Scale V.1.1
Sep 6th • 17 mins read

Professional Medical Writer Assistance in Oncology Clinical Trials
Sep 17th • 7 mins read
Quantitative Clinical Pharmacology of T‐Cell Engaging Bispecifics: Current Perspectives and Opportunities
Nov 18th • 15 mins read
Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration
Aug 31st • 16 mins read

Past, Current, and Future Cancer Clinical Research Collaborations: The Case of the European Organisation for Research and Treatment of Cancer
Aug 16th • 8 mins read

Pediatric Oncology Clinical Trials and Collaborative Research in Africa: Current Landscape and Future Perspectives
Aug 7th • 10 mins read

Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration
Jul 22nd • 12 mins read
Comparison of Access to Novel Drugs for Lymphoma and Chronic Lymphocytic Leukemia Between India and the United States
Jul 21st • 12 mins read
Quantitative Translation in Immuno-Oncology Research and Development
Jul 9th • 3 mins read

Biosimilars in oncology: key role of nurses in patient education
Jun 15th • 10 mins read
Tumor Growth Dynamic Modeling in Oncology Drug Development and Regulatory Approval: Past, Present, and Future Opportunities
Jun 26th • 18 mins read

Clinical development success rates and social value of pediatric Phase 1 trials in oncology
Jun 21st • 28 mins read
Characterizing Exposure–Response Relationship for Therapeutic Monoclonal Antibodies in Immuno-Oncology and Beyond: Challenges, Perspectives, and Prospects
Jun 18th • 30 mins read

Real-World Evidence: Bridging Gaps in Evidence to Guide Payer Decisions
Jun 18th • 6 mins read

Comment on: Oncology research in Saudi Arabia over a 10-year period. A synopsis
Jun 24th • 3 mins read

The regulatory landscape of precision oncology laboratory medicine in the United States - Perspective on the past 5 years and considerations for future regulation
May 22nd • 8 mins read

Sponsorship of oncology clinical trials in the United States according to age of eligibility
Apr 29th • 8 mins read

The Oncology Data Network (ODN): Methodology, Challenges, and Achievements
May 21st • 8 mins read

Assessment of Clinical Trials Supporting US Food and Drug Administration Approval of Novel Therapeutic Agents, 1995-2017
Apr 21st • 20 mins read

Proportion of Patients in Phase I Oncology Trials Receiving Treatments That Are Ultimately Approved
Apr 1st • 14 mins read
Access to Novel Drugs for Non-Small Cell Lung Cancer in Central and Southeastern Europe: A Central European Cooperative Oncology Group Analysis
Nov 24th • 10 mins read

Publicly accessible evidence of health-related quality of life benefits associated with cancer drugs approved by the European Medicines Agency between 2009 and 2015
Feb 23rd • 12 mins read

Patient burden and clinical advances associated with post approval monotherapy cancer drug trials: a retrospective cohort study
Feb 17th • 7 mins read
In Vitro-to-In Vivo Extrapolation of Transporter Inhibition Data for Drugs Approved by the US Food and Drug Administration in 2018
Jan 25th • 12 mins read
EHA evaluation of the ESMO-Magnitude of Clinical Benefit Scale version 1.1 (ESMO-MCBS v1.1) for hematological malignancies
Jan 20th • 20 mins read
Precision medicine for pediatric cancers lags behind that for adult cancers: Citing hurdles in clinical trial recruitment and drug development, researchers are exploring ways to close gaps
Jan 9th • 2 mins read

Developing a framework to incorporate real-world evidence in cancer drug funding decisions: the Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue) collaboration
Jan 7th • 8 mins read
Rise of Antibody-Drug Conjugates: The Present and Future
May 25th • 20 mins read

The pitfalls and promise of liquid biopsies for diagnosing and treating solid tumors in children: a review
Jan 3rd • 10 mins read

Real-World Evidence in Oncology: Opportunities and Limitations
Dec 24th • 8 mins read

Outcome measures for oncology alternative payment models: practical considerations and recommendations
Dec 1st • 10 mins read

Value assessment of oncology drugs using a weighted criterion-based approach
Dec 20th • 15 mins read

Mandatory Research Biopsy Requirements Delay Initiation of Clinical Trials
Oct 18th • 10 mins read
Pivotal Considerations for Optimal Deployment of Healthy Volunteers in Oncology Drug Development
Oct 31st • 20 mins read
Publication statuses of clinical trials supporting FDA-approved immune checkpoint inhibitors: a meta-epidemiological investigation
Oct 24th • 18 mins read

First person profile: Supriya G. Mohile, MD, MS: A respected geriatric oncologist, Dr. Mohile has conducted innovative research on improving care for older patients with cancer
Oct 11th • 2 mins read
New Realities of Phase I Clinical Trials in the Era of
Oct 7th • 5 mins read

Liquid biopsy in oncology: a consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology
Sep 26th • 16 mins read

Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2
Sep 20th • 12 mins read

Association of National Cancer Institute-Sponsored Clinical Trial Network Group Studies With Guideline Care and New Drug Indications
Sep 4th • 17 mins read

The rise of oncology biosimilars: from process to promise
Aug 23rd • 18 mins read
Early-drug development in the era of immuno-oncology: are we ready to face the challenges?
Jun 26th • 26 mins read

First person profile: Leslie Bernstein, PhD: An epidemiologist known for her groundbreaking discoveries about breast cancer, Dr. Bernstein continues to push the field forward
Aug 12th • 4 mins read

Level of evidence used in recommendations by the National Comprehensive Cancer Network (NCCN) guidelines beyond Food and Drug Administration approvals
Aug 2nd • 8 mins read

Median Survival or Mean Survival: Which Measure Is the Most Appropriate for Patients, Physicians, and Policymakers?
Jul 18th • 15 mins read

Cost per Event Averted in Cancer Trials in the Adjuvant Setting From 2018 to 2022
Jun 10th • 30 mins read

Comparison of Long-term Survival Benefits in Trials of Immune Checkpoint Inhibitor vs Non-Immune Checkpoint Inhibitor Anticancer Agents Using ASCO Value Framework and ESMO Magnitude of Clinical Benefit Scale
Jul 10th • 12 mins read

Clinical benefit of cancer drugs approved in Switzerland 2010–2019
Jun 10th • 35 mins read

A Comprehensive Comparison of Additional Benefit Assessment Methods Applied by Institute for Quality and Efficiency in Health Care and European Society for Medical Oncology for Time-to-Event Endpoints After Significant Phase III Trials—A Simulation Study
Jun 28th • 30 mins read

Uptake of Oncology Biosimilars: Managed Care Strategies to Improve Value-Based Care Systems
Jul 7th • 25 mins read

Audit of Data Sharing by Pharmaceutical Companies for Anticancer Medicines Approved by the US Food and Drug Administration
Jul 28th • 20 mins read

Overall Survival Benefits of Cancer Drugs Approved in China From 2005 to 2020
Aug 10th • 30 mins read
Recent Trends in Medicaid Spending and Use of Drugs With US Food and Drug Administration Accelerated Approval
Oct 8th • 25 mins read

How do cancer clinicians perceive real-world data and the evidence derived therefrom? Findings from an international survey of the European Organisation for Research and Treatment of Cancer
Aug 1st • 45 mins read

FDA validation of surrogate endpoints in oncology: 2005–2022
Dec 1st • 20 mins read


Oncology biosimilars: New developments and future directions
Nov 25th • 30 mins read

Association Between US Drug Price and Measures of Efficacy for Oncology Drugs Approved by the US Food and Drug Administration From 2015 to 2020
Oct 31st • 10 mins read
Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption
Oct 27th • 30 mins read

Association between control group therapy and magnitude of clinical benefit of cancer drugs
Dec 9th • 20 mins read

Methodological and reporting standards for quality-of-life data eligible for European Society for Medical Oncology-Magnitude of Clinical Benefit Scale
Apr 1st • 30 mins read

Biosimilars in Oncology: Latest Trends and Regulatory Status
Dec 5th • 20 mins read

Towards a novel approach guiding the decision-making process for anticancer treatment in patients with advanced cancer: framework for systemic anticancer treatment with palliative intent
Jun 1st • 25 mins read

Patient involvement: A must-have in medicine development, but is it being overlooked in a cost-constrained environment?
May 9th • 5 mins read

Medical affairs: The power behind redefining commercialization
Aug 2nd • 3 mins read

Empowering people to drive medical affairs performance with AI
Aug 14th • 5 mins read
Exposure to US Cancer Drugs With Lack of Confirmed Benefit After US Food and Drug Administration Accelerated Approval
Dec 8th • 2 mins read

The Value of Pharmaceutical Industry-Sponsored Patient Registries in Oncology Clinical Research
Jun 7th • 8 mins read
Value assessment of PD-1/PD-L1 inhibitors in the treatment of esophageal and gastrointestinal cancers
Apr 21st • 13 mins read

Single-arm trials supporting the approval of anticancer medicinal products in the European Union: contextualization of trial results and observed clinical benefit
Apr 11th • 14 mins read

Defining the role of real-world data in cancer clinical research: The position of the
Mar 20th • 10 mins read

Evaluating External Validity of Oncology Biosimilar Safety Studies
Apr 6th • 2 mins read
Preliminary Attainability Assessment of Real-World Data for Answering Major Clinical Research Questions in Breast Cancer Brain Metastasis: Framework Development and Validation Study
Oct 9th • 4 mins read

The correlation between the costs and clinical benefits of PD-1/PD-L1 inhibitors in malignant tumors: An evaluation based on ASCO and ESMO frameworks
Feb 23rd • 9 mins read

Value assessment of NMPA-approved new cancer drugs for solid cancer in China, 2016-2020
Feb 24th • 8 mins read
Early phase clinical trial played a critical role in the Food and Drug Administration-approved indications for targeted anticancer drugs: a cross-sectional study from 2012 to 2021
Mar 9th • 10 mins read

Off-label despite high-level evidence: a clinical practice review of commonly used off-patent cancer medicines
Nov 14th • 21 mins read

US Government Payer-Funded Trials to Address Oncology's Drug-Dosing Conundrum: A Congressional Call to Action?
Feb 13th • 5 mins read

What is the weight of expectation bias in oncology trials?
Feb 11th • 3 mins read
Factors associated with the uptake of biosimilars for breast cancer treatment from the perspectives of physicians and patients-Evidence from China
Jan 12th • 13 mins read

Twelve ESMO Congress 2022 breakthroughs: practicing oncologists' perceptions and potential application on presented data
Jan 10th • 12 mins read

Medical Oncologists’ Knowledge and Perspectives on the Use of Biosimilars in the United States
Jan 9th • 9 mins read
Application of Value Framework to Phase III Trials of Immune Checkpoint Inhibitors in Esophageal and Gastric Cancer
Jan 13th • 8 mins read

Embracing Project Optimus: Can we Leverage Evolutionary Theory to Optimize Dosing in Oncology?
Sep 2nd • 10 mins read

Are the chronological age cutoffs used in clinical oncology guidelines biologically meaningful?
Dec 1st • 4 mins read

The challenges and opportunities in using real-world data to drive advances in healthcare in East Asia: expert panel recommendations
Jun 28th • 13 mins read

Rationale, Strengths, and Limitations of Real-World Evidence in Oncology: A Canadian Review and Perspective
Apr 26th • 9 mins read
Building a Healthcare Alliance for Resourceful Medicine Offensive Against Neoplasms in Hematology Added Value Framework for Hematologic Malignancies: A Comparative Analysis of Existing Tools
May 17th • 12 mins read

External control arms in oncology: current use and future directions
Jan 9th • 9 mins read

CheckMate-067: Raising the Bar for the Next Decade in Oncology
Dec 2nd • 2 mins read

Report from American Society of Clinical Oncology Symposium 2020 and American Society of Clinical Oncology Gastrointestinal Cancer Symposium 2021
Aug 4th • 13 mins read

Real-World Evidence in Support of Oncology Product Registration: A Systematic Review of New Drug Application and Biologics License Application
Jan 1st • 12 mins read

Estimated Medicare Spending on Cancer Drug Indications With a Confirmed Lack of Clinical Benefit After US Food and Drug Administration Accelerated
Oct 18th • 5 mins read

Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: retrospective observational study
Aug 4th • 12 mins read

The Inclusion of Women in Global Oncology Drug Trials Over the Past 20 Years
Aug 26th • 2 mins read
Implications of Research Biopsies in Clinical Trials
Aug 31st • 2 mins read
Canadian Regulatory and Health Technology Assessment for Malignant Hematology and Oncology Indications Compared With the US Food and Drug Administration Accelerated Approval Program
Jun 5th • 6 mins read

Payer perceptions of the use of real-world evidence in oncology-based decision making
Aug 1st • 12 mins read

Assessment of gender representation in clinical trials leading to FDA approval for oncology therapeutics between 2014 and 2019: A systematic review-based cohort study
Jun 23rd • 8 mins read

Use of real-world evidence for oncology clinical decision making in emerging economies
May 5th • 12 mins read

Patient Participation in Clinical Trials of Oncology Drugs and Biologics Preceding Approval by the US Food and Drug Administration
May 18th • 5 mins read

Patient-Reported Outcomes in Pediatric Cancer Registration Trials: A US Food and Drug Administration Perspective
Apr 30th • 12 mins read
Model-Informed Therapeutic Dose Optimization Strategies for Antibody-Drug Conjugates in Oncology: What Can We Learn From US Food and Drug Administration-Approved Antibody-Drug Conjugates?
Apr 26th • 15 mins read

Oncology approvals in 2020: a year of firsts in the midst of a pandemic
Jan 29th • 4 mins read

FDA Oncology Center of Excellence Project Renewal: Engaging the Oncology Community to Update Product Labeling for Older Oncology Drugs
Feb 15th • 13 mins read
Inadequate and delayed characterization of cutaneous reactions for US Food and Drug Administration-approved oncologic drugs from 2011-2020 leading to medication discontinuation
Oct 23rd • 4 mins read

Use of real-world evidence in cancer drug funding decisions in Canada: a qualitative study of stakeholders’ perspectives
Nov 4th • 12 mins read

The Evidence REVEAL Study: Exploring the Use of Real-World Evidence and Complex Clinical Trial Design by the European Pharmaceutical Industry
Nov 20th • 10 mins read

Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016
Nov 10th • 6 mins read

Potential Cost Implications for All US Food and Drug Administration Oncology Drug Approvals in 2018
Aug 31st • 7 mins read

Use of Real-World Evidence to Support FDA Approval of Oncology Drugs
Sep 14th • 16 mins read

Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States
Oct 6th • 48 mins read
Discordance Between Child-Pugh and National Cancer Institute Classifications for Hepatic Dysfunction: Implications on Dosing Recommendations for Oncology Compounds
Jul 20th • 18 mins read
Mechanistic Quantitative Pharmacology Strategies for the Early Clinical Development of Bispecific Antibodies in Oncology
Jun 24th • 18 mins read
Sponsorship of oncology clinical trials in the United States according to age of eligibility
Apr 29th • 6 mins read
FDA Acceptance of Surrogate End Points for Cancer Drug Approval: 1992-2019
Mar 9th • 4 mins read

The Use of Not-Negative Conclusions to Describe Results of Formally Negative Trials Presented at Oncology Meetings
Feb 13th • 2 mins read

Biased by design? Clinical trials and patient benefit in oncology
Nov 27th • 3 mins read

Pediatric Trials for Cancer Therapies With Targets Potentially Relevant to Pediatric Cancers
Oct 29th • 10 mins read

Defining a Clinically Meaningful Benefit in Cancer Clinical Trials: From the Perspectives of the Clinical Trialist, Patient, and Society
Jun 27th • 2 mins read
Dose Finding in the Clinical Development of 60 US Food and Drug Administration-Approved Drugs Compared With Learning vs. Confirming Recommendations
Jun 29th • 15 mins read

Addressing oncologists' gaps in the use of biosimilar products
Jun 19th • 5 mins read
The evolving landscape of precision medicine in primary liver cancer
Mar 29th • 3 mins read
Assessing cardiac safety in oncology drug development
Jun 12th • 12 mins read

Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer
Jun 6th • 2 mins read

An Overview of Cancer Drugs Approved by the US Food and Drug Administration Based on the Surrogate End Point of Response Rate
May 28th • 5 mins read
Quantitative Mechanistic Modeling in Support of Pharmacological Therapeutics Development in Immuno-Oncology
Apr 30th • 12 mins read

Assessment of Whether the American Society of Clinical Oncology's Value Framework and the European Society for Medical Oncology's Magnitude of Clinical Benefit
May 16th • 15 mins read
Association of Industry and Academic Sponsorship With Negative Phase 3 Oncology Trials and Reported Outcomes on Participant Survival: A Pooled Analysis
May 10th • 8 mins read
Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs
May 3rd • 10 mins read

Analysis of Control Arm Quality in Randomized Clinical Trials Leading to Anticancer Drug Approval by the US Food and Drug Administration
May 2nd • 15 mins read
Cancer drug development: The missing links
Apr 18th • 20 mins read

Cancer experts point to new advances in research and treatment: A recent report by the American Association for Cancer Research highlights unprecedented successes as well as ongoing challenges in the cancer field
Apr 1st • 2 mins read

Biosimilars: what the oncologist should know
Dec 18th • 25 mins read

Timing of first-in-child trials of FDA-approved oncology drugs
Mar 18th • 10 mins read
Estimation of Study Time Reduction Using Surrogate End Points Rather Than Overall Survival in Oncology Clinical Trials
Apr 1st • 10 mins read
Representation of Patients With Cardiovascular Disease in Pivotal Cancer Clinical Trials
Mar 18th • 3 mins read
Anticancer drugs approved by the Food and Drug Administration for gastrointestinal malignancies: Clinical benefit and price considerations
Mar 7th • 8 mins read

Value assessment frameworks in oncology: championing concordance through shared standards
Feb 18th • 1 min read
The Case for Real-world Evidence in the Future of Clinical Research on Chronic Myeloid Leukemia
Jan 29th • 10 mins read
Overview of Oncology and Hematology Drug Approvals at US Food and Drug Administration Between 2008 and 2016
Aug 4th • 15 mins read

Opportunities and challenges in biosimilar uptake in oncology
Jun 26th • 7 mins read

Cancer research in the United States: A critical review of current status and proposal for alternative models
May 14th • 10 mins read
Reverse Translation of US Food and Drug Administration Reviews of Oncology New Molecular Entities Approved in 2011-2017: Lessons Learned for Anticancer Drug Development
Mar 11th • 15 mins read
Challenges and Opportunities in Dose Finding in Oncology and Immuno-oncology
Jul 11th • 10 mins read

Evolving Landscape of US Food and Drug Administration Drug Approval in the Era of Precision Oncology: Finding the Right Balance Between Access and Safety
Jun 20th • 3 mins read

The FDA Oncology Center of Excellence and Precision Medicine
Nov 6th • 3 mins read

Streamlining Adverse Events Reporting in Oncology: An American Society of Clinical Oncology Research Statement
Feb 20th • 3 mins read

Magnitude of Clinical Benefit of Cancer Drugs Approved by the US Food and Drug Administration
Dec 13th • 7 mins read

AI-powered real-world evidence: Strategically enhancing value and access
Aug 22nd • 5 mins read


